
Galvani's voltaic pile Science Museum Group Collection
1 LEARN ABOUT THESE METRICS Share Export RIS PDF (1 MB) Get e-Alerts Abstract An inexpensive, simple, and fun way to illustrate many of the principles in electrochemistry. KEYWORDS (Audience): First-Year Undergraduate / General KEYWORDS (Domain): Demonstrations KEYWORDS (Feature): Tested Demonstrations KEYWORDS (Subject): Electrochemistry Cited By

Voltaic Pile In A Class Room Stock Photo Download Image Now Stack, Electricity, Laboratory
1 Introduction This is a story describing the challenge and entertainment of restaging the experiment to decompose water using electric energy from a voltaic pile in a history of science course.
Voltaic Pile ClipArt ETC
Make a battery with pennies, nickels, salt, and vinegar in this fun science experiment! This type of battery is also called a voltaic pile. You can use a mul.
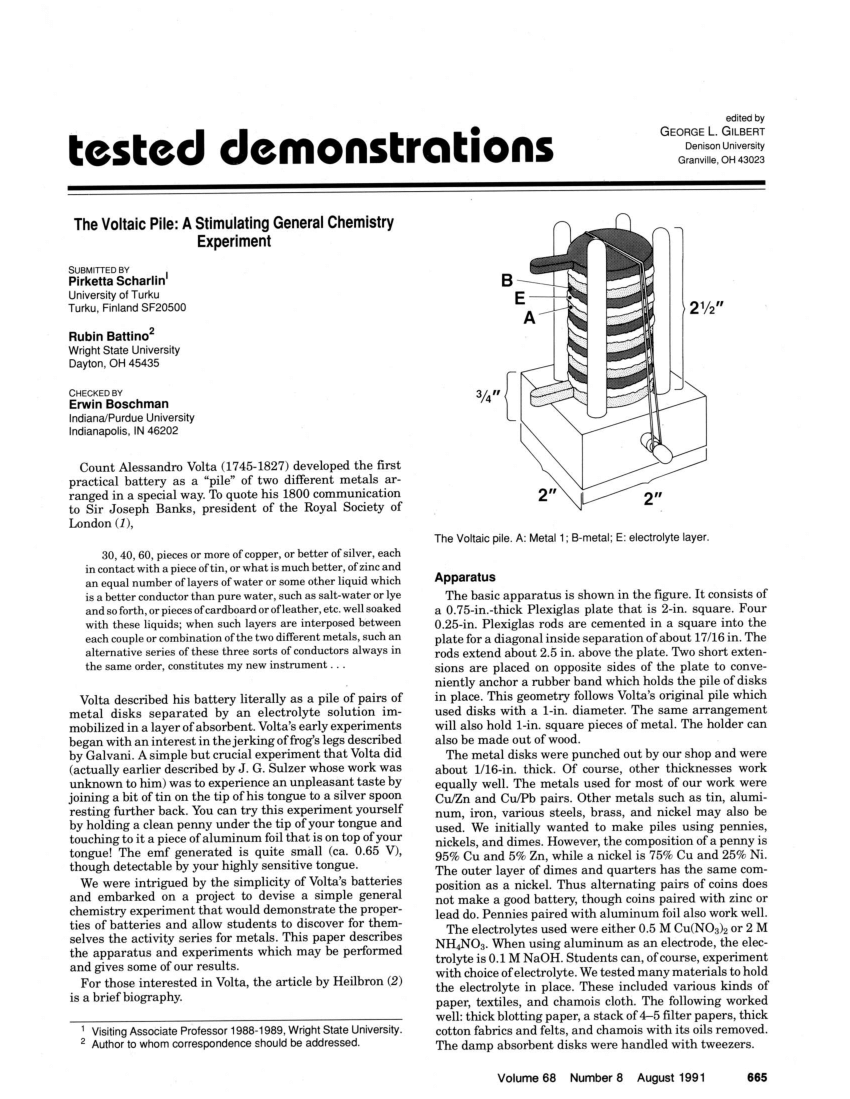
(PDF) The Voltaic pile A stimulating general chemistry experiment
The finding led him to experiment with replacing the oil in lamps with methane and to create the Volta lamplighter. He rejects animal electricity. During those years, the inventor also travelled through Europe and came into contact with renowned intellectuals of the time, such as Horace-Bénédict de Saussure and Voltaire.. The voltaic pile.

Voltaic pile by Newton & Co. Science Museum Group Collection
The voltaic pile was the first electrical battery that could continuously provide an electric current to a circuit. It was invented by Italian chemist Alessandro Volta, who published his experiments in 1799.

Making Voltaic Batteries in Mum's Kitchen News about Energy Storage, Batteries, Climate Change
In 1800 Italian Physicist Alessandro Volta developed the voltaic pile, a forerunner of the electric battery. The first pile consisted of a number of discs of zinc and silver separated by pieces of wet paper and arranged in a vertical column.

Voltaic Pile Science History Institute Digital Collections
To construct a series of voltaic cells powerful enough to do visible work. Volta used zinc and silver; we will use zinc and more easily available copper. Materials 1. A strip of aluminum foil 2. About five or six pieces of 1-inch square cardboard or card stock saturated with sodium chloride solution 3. About five or six pennies (copper shell) 4.
Battery Voltaic Pile National Museum of American History
A voltaic pile is an early form of electric battery. Italian physicist Alessandro Volta stacked piles of alternating metal copper and zinc discs separated by pieces of cloth or cardboard soaked in an electrolyte solution.

Experiment Make a Voltaic Pile News about Energy Storage, Batteries, Climate Change and the
This is also called a voltaic pile, which is named after Alessandro Volta, who created the first battery in 1800 by alternating zinc and copper electrodes with sulfuric acid between them. In Volta's battery and your penny battery, an oxidation reaction occurs at the zinc electrode that releases electrons and a reduction reaction occurs at the.

Original Voltaic pile. Probably made and used by Volta himself. This pile was shown at the
The Voltaic Pile and its Consequences. The next great step forward can be attributed to Alessandro Volta, who in 1799, following close upon the discovery of the galvanic effect by Galvani, built the first electric battery. This immediately became known as Volta's Pile and, like many other batteries which followed it, employed copper as an.

"Plating out." Química, Aula de química, Clase de química
The Voltaic pile: A stimulating general chemistry experiment Journal of Chemical Education Authors: Pirketta Scharlin Rubin Battino Wright State University Abstract An inexpensive, simple, and.

Voltaic pile invented by the italian physicist Alessandro Volta (17451827). Colored engraving
You can make your own voltaic pile out of simple and inexpensive materials. Although Volta used silver and zinc, it is more feasible - and inexpensive - to use copper and zinc for the metal disks.
Alessandro Volta The Voltaic Pile Experiment The Invention of the Electric Battery
The Voltaic Pail Experiment Basically, Volta's pile was a messy stack (pile) of discs made of two types of metal - one silver, the other zinc. The discs were separated from each other by a piece of cloth or cardboard that had been soaked in salt water (brine).

Reproduction of Voltaic Pile Science History Institute Digital Collections
Steps: Cut the aluminum foil and cardboard into circles. Set the cut outs aside. Make an acid mixture by mixing cider vinegar and salt in a saucer. Soak the cut out cardboard in the acid mixture. Tape the copper wire to one of the cut out aluminum foils. Alternately stack the aluminum foil, cardboard and coin.
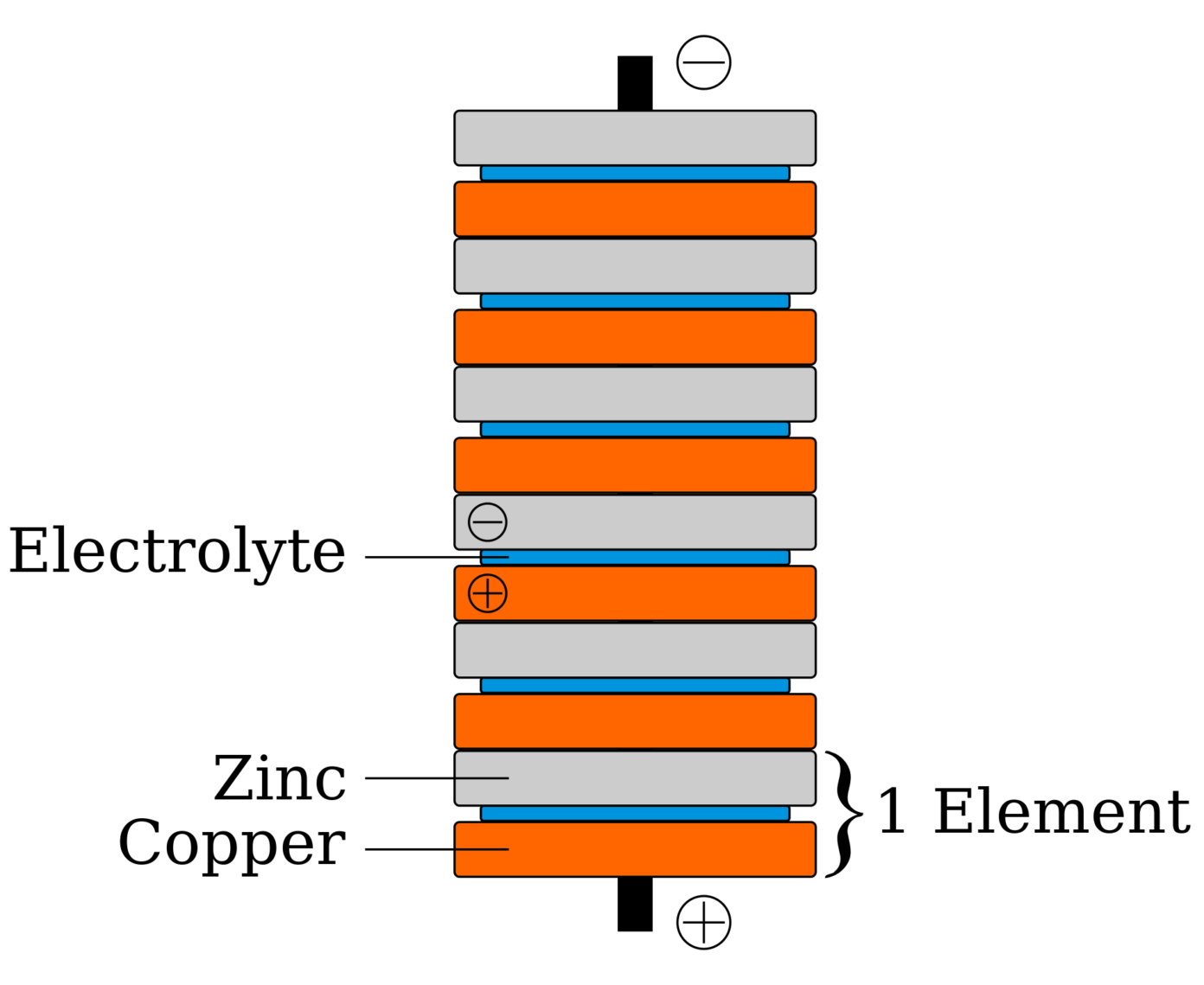
The Voltaic pile BatteryIndustry.tech
Watch as Dr. Joel Bryan of Ball State University discusses the history of the voltaic pile and shows how you can make one yourself out of simple and inexpensive materials.

compensar Menstruación cascada alessandro volta pila Beber agua Labe transacción
In this experiment, you will make your own voltaic pile using pennies and nickels. How many coins in the pile will make the most electricity? Summary Areas of Science Energy & Power Difficulty Time Required Very Short (≤ 1 day) Prerequisites To do this project, you will need an adult to help you use a multimeter.